What’s in Kim’s Mailbox?
I’m back at you today with a fresh new breakdown of an “O to O” promo that landed in my (physical) mailbox this morning.
You may ask what I mean by “O to O”? It’s “offline to online”… and I’m seeing more of these direct mail promotions over the past year or so.
They tend to be simple, tri-fold self-mailers on heavy card stock, folded down to the postage-efficient 6×9 flat size (for the lowest rates).
They’re big on graphics, not so heavy on copy, and unlike most long-form direct mail promos, their purpose is to drive prospects online to complete the selling process and order.
The promo I’m going to share with you today jumps out because it’s a perfect example of the kind of copy that can land you or your client in FDA “jail”.
Meaning… it’s stunningly non-compliant!
As far as I know, the rules haven’t changed: you can’t use disease names in your copy. The only exception would be if the FDA has reviewed and approved a specific Qualified Health Claim.
To my knowledge, that has NOT been done for probiotic supplements like the one this mailing is promoting. And definitely NOT for a Type 2 diabetes claim!
I went online to investigate further, since I thought maybe it’s actually a drug… but it’s not (though based on its price, it might as well be!)
Let’s take a look at the front of this promo and you’ll see what I’m talking about…

Whaaat? After nearly three decades of marketing nutritional supplements, arguing with clients and lawyers about “risk tolerance”, and neutering my copy to please the FDA and FTC Gods, I just about fell out of my chair when I saw “diabetes” in big bold print.
(I wish I could say that’s how I broke my pinky toe last Friday… but it was a sleepy walk to the bathroom colliding with the big white chair that my dog Pearl likes to sleep in that did it!)
Now, MAYBE they think they’re being compliant by using such a boring-a*s headline. After all, it’s not claiming to “diagnose, treat, cure, or prevent any disease”… as the required FDA disclosure that’s required on direct mail supplement promos (and is flagrantly missing here) says.
But “manage” ain’t the problem… it’s using the actual DISEASE name that is! Had they simply said, “Help your body manage your blood sugar” or “Help your body take control of your blood sugar”, they’d be playing it reasonably safe.
The rest of the copy is mostly “ad agency”-lame as well… meaning, in love with itself. When you open up the front panel, here’s what the inside looks like…
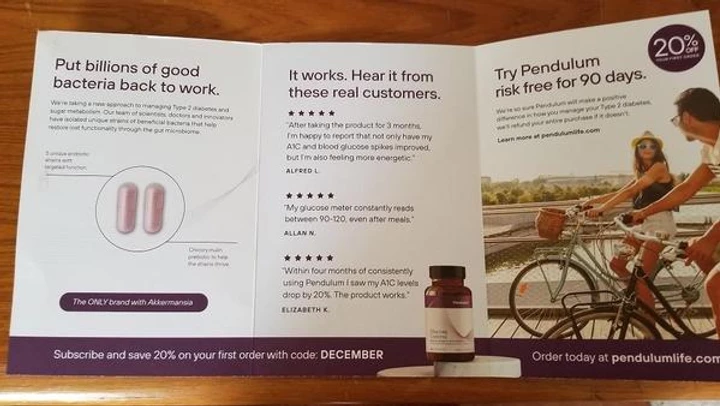
“Put billions of good bacteria back to work”? What does that even mean? I didn’t know they were out of a job. When you are using such a small amount of copy for your sales message, every word counts. (Every word counts if it’s a 40-page promo, too!)
And while it may be hard to read, the paragraph under that confusing and uninspiring header is about all about THEM… not the reader. “We’re taking a new approach…” “Our team of scientists…” blah blah blah
No mention of studies, research, or proof. A few nicely-placed customer testimonials, but that’s it. I expect a little science here. After all, you’re talking about a condition most people treat with medication. This is a product that has serious consequences for your health. If had diabetes, I’d definitely want to know it works and it’s safe.
On the right hand side, there’s a strong 90-day guarantee and an almost-missable callout about a 20% discount (it’s also fairly prominent across the bottom). The call to action takes you to their website at pendulumlife.com.
So I decided to go there. But first, let me share one of the inner panels with you so you can see just how outright egregious they were about throwing the no-no word “diabetes” around (it’s on the back panel, too):

I get it… it’s a “medical probiotic”! Sorry, Charlie, that’s still not going to fly with the FDA.
You can check to see which Qualified Claims they’ve reviewed (and mostly did NOT approve) here. I’m no FDA lawyer, but I don’t see any here for diabetes and “medical” probiotics!
So after featuring this one product (with the imaginative name, “Glucose Control”) in the promo, when you go to the website the promo sends you to, you are now faced with TWO choices…
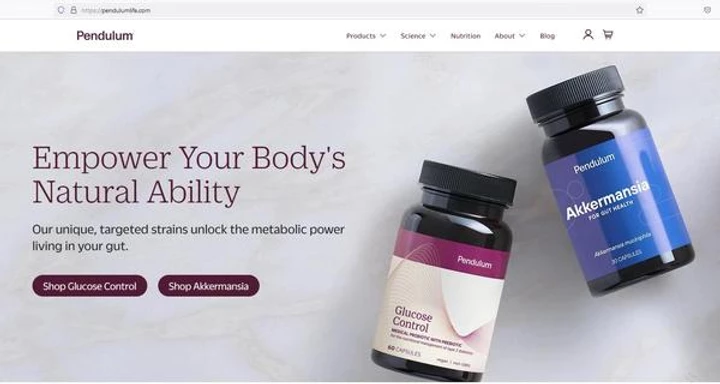
One of the cardinal rules of direct response copy is to NEVER confuse the prospect, especially at the critical point of ordering!
And there’s nothing here in the copy about Diabetes (guess they thought they could get away with it in the mail but not online) nor blood sugar even. (Oh wait! When you scroll down they do mention “Type 2 Diabetes”. Definitely living on the edge here…)
Now for another thing that made me almost fall out of my chair: the price! Here’s what you see when you click the button for the Glucose Control product…
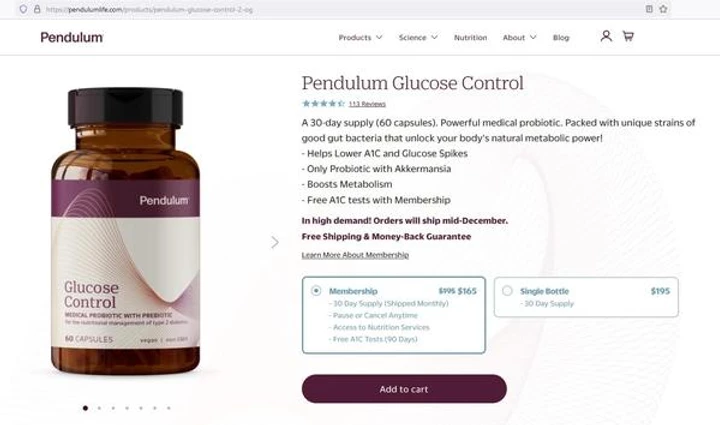
If you can’t read it, just click on the image above and it will take you to the page. And you can fall out of your chair, too! Now, you know that profit margin has got to be HUUUUUUgeeee…
Guess that moo-lah will come in handy to pay the attorney’s fees when they get the inevitable warning letter from the FDA (just like these companies promoting “diabetes” supplements did recently). And that will force them to stop promoting at least for a period of time. Not something any business wants to open themselves up to.
And it’s really not needed in order to sell a blood sugar-supporting supplement. So avoid disease names in your supplement copy, they’re just not worth it!
Yours for smarter marketing,
Kim
P.S. If you like these kinds of breakdowns, you’ll go crazy over seeing me LIVE breaking down in detail one of my successful control promos. You’ll have that chance to join me this coming Friday. Check out all the details here on grabbing a front-row seat!